 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
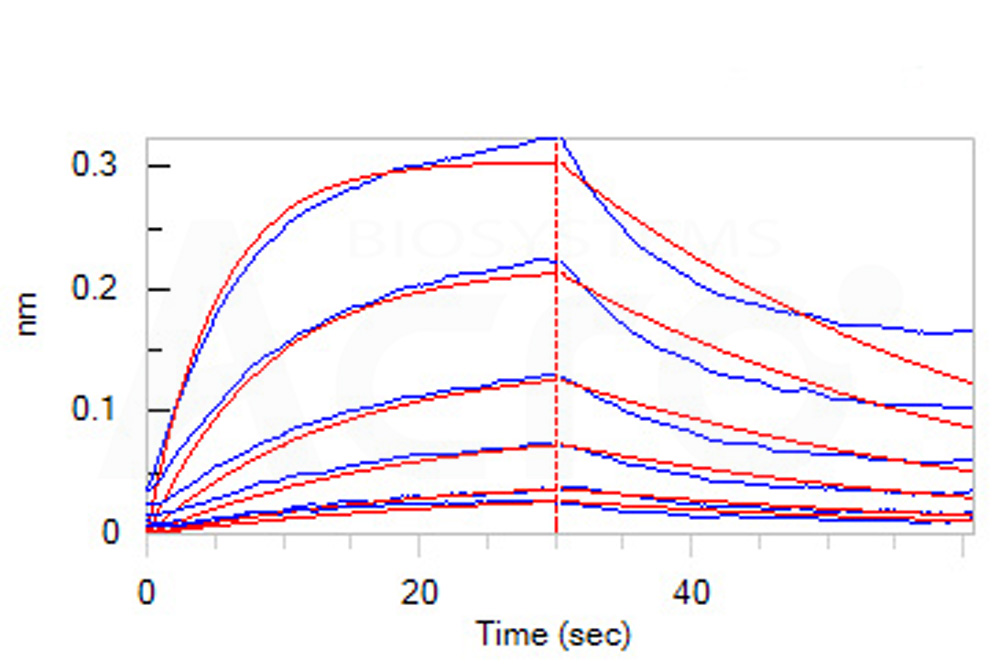
Loaded Human IL-6 R alpha, Fc Tag (Cat. No. ILR-H5259) on Protein A Biosensor, can bind Human IL-6, premium grade (Cat. No. IL6-H4218) with an affinity constant of 35.9 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Tocilizumab | rhPM-1; RO-48775533; RG-1569; MRA; HPM-1; MRA-SC; R-1569 | Approved | Chugai Pharmaceutical Co Ltd | Actemra, RoActemra, 雅美罗, Actemra/RoActemra, Acterma | Japan | Arthritis, Rheumatoid; Giant Cell Arteritis; Takayasu Arteritis; Cytokine Release Syndrome; Multicentric Castleman's Disease (MCD); Arthritis, Juvenile | null | 2005-04-11 | Uveitis, Posterior; Polymyalgia Rheumatica; Takayasu Arteritis; Multicentric Castleman's Disease (MCD); systemic sclerosis-associated interstitial lung disease; Breast Neoplasms; Coronavirus Infections; Lupus Erythematosus, Systemic; Coronary Disease; Pulmonary Arterial Hypertension; Fever; Coronaviridae Infections; Lymphoma; Pleural Effusion, Malignant; Stroke; Inflammation; Rejection in heart transplantation; Pulmonary Disease, Chronic Obstructive; Uveitis; Osteoarthritis; Panuveitis; Non-ST Elevated Myocardial Infarction; Carcinoma, Hepatocellular; Anemia, Sickle Cell; Diabetes Mellitus; Behcet Syndrome; Amyotrophic Lateral Sclerosis; Kidney Failure, Chronic; Myocardial Infarction; Solid tumours; Non-radiographic axial spondyloarthritis; Cytokine Release Syndrome; HIV Infections; Acute Chest Syndrome; Diabetes Mellitus, Type 1; Craniopharyngioma; Motor Neuron Disease; Polymyositis; Schnitzler Syndrome; Giant Cell Arteritis; Uveitis, Intermediate; Pneumonia; Hematologic Neoplasms; Polyarticular Juvenile Idi | Details |
| Tocilizumab biosimilar (Bio-Thera Solutions) | BAT-1806; BIIB-800 | Approved | Bio-Thera Solutions Ltd | 施瑞立, TOFIDENCE | Mainland China | Arthritis, Rheumatoid; Arthritis, Juvenile; Cytokine Release Syndrome | Bio-Thera Solutions Ltd | 2023-01-16 | Cytokine Release Syndrome; Polyarticular Juvenile Idiopathic Arthritis; Arthritis, Rheumatoid; Arthritis, Juvenile | Details |
| Satralizumab | RG-6168; SA-237 | Approved | Chugai Pharmaceutical Co Ltd | Enspryng, エンスプリング | Japan | Neuromyelitis Optica | Chugai Pharmaceutical Co Ltd | 2020-06-29 | Myasthenia Gravis; Brain Ischemia; Graves Ophthalmopathy; Neuromyelitis Optica; Pulmonary Arterial Hypertension; Encephalitis; Subarachnoid Hemorrhage; Anti-N-Methyl-D-Aspartate Receptor Encephalitis | Details |
| Sarilumab | SAR-153191; REGN-88 | Approved | Sanofi | Kevzara | United States | Arthritis, Rheumatoid | Sanofi-Synthelabo | 2017-05-22 | Non-radiographic axial spondyloarthritis; Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19); Sarcoidosis; Mastocytosis, Systemic; Arthritis, Juvenile; Polymyalgia Rheumatica; Severe Acute Respiratory Syndrome; Uveitis | Details |
| Tocilizumab biosimilar (Livzon Group) | LZM-008 | Approved | Livzon Pharmaceutical Group Inc | 安维泰 | Mainland China | Arthritis, Rheumatoid | Livzon Mabpharm Inc | 2023-01-18 | Arthritis, Rheumatoid | Details |
| Tocilizumab biosimilar (Fresenius kabi) | MSB-11456 | Approved | Merck Serono | Tyenne® | EU | Cytokine Release Syndrome; Arthritis, Rheumatoid; Giant Cell Arteritis; Coronavirus Disease 2019 (COVID-19) | Fresenius Kabi Deutschland Gmbh | 2023-09-15 | Cytokine Release Syndrome; Giant Cell Arteritis; Polyarticular Juvenile Idiopathic Arthritis; Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19) | Details |
| Tocilizumab | rhPM-1; RO-48775533; RG-1569; MRA; HPM-1; MRA-SC; R-1569 | Approved | Chugai Pharmaceutical Co Ltd | Actemra, RoActemra, 雅美罗, Actemra/RoActemra, Acterma | Japan | Arthritis, Rheumatoid; Giant Cell Arteritis; Takayasu Arteritis; Cytokine Release Syndrome; Multicentric Castleman's Disease (MCD); Arthritis, Juvenile | null | 2005-04-11 | Uveitis, Posterior; Polymyalgia Rheumatica; Takayasu Arteritis; Multicentric Castleman's Disease (MCD); systemic sclerosis-associated interstitial lung disease; Breast Neoplasms; Coronavirus Infections; Lupus Erythematosus, Systemic; Coronary Disease; Pulmonary Arterial Hypertension; Fever; Coronaviridae Infections; Lymphoma; Pleural Effusion, Malignant; Stroke; Inflammation; Rejection in heart transplantation; Pulmonary Disease, Chronic Obstructive; Uveitis; Osteoarthritis; Panuveitis; Non-ST Elevated Myocardial Infarction; Carcinoma, Hepatocellular; Anemia, Sickle Cell; Diabetes Mellitus; Behcet Syndrome; Amyotrophic Lateral Sclerosis; Kidney Failure, Chronic; Myocardial Infarction; Solid tumours; Non-radiographic axial spondyloarthritis; Cytokine Release Syndrome; HIV Infections; Acute Chest Syndrome; Diabetes Mellitus, Type 1; Craniopharyngioma; Motor Neuron Disease; Polymyositis; Schnitzler Syndrome; Giant Cell Arteritis; Uveitis, Intermediate; Pneumonia; Hematologic Neoplasms; Polyarticular Juvenile Idi | Details |
| Tocilizumab biosimilar (Bio-Thera Solutions) | BAT-1806; BIIB-800 | Approved | Bio-Thera Solutions Ltd | 施瑞立, TOFIDENCE | Mainland China | Arthritis, Rheumatoid; Arthritis, Juvenile; Cytokine Release Syndrome | Bio-Thera Solutions Ltd | 2023-01-16 | Cytokine Release Syndrome; Polyarticular Juvenile Idiopathic Arthritis; Arthritis, Rheumatoid; Arthritis, Juvenile | Details |
| Satralizumab | RG-6168; SA-237 | Approved | Chugai Pharmaceutical Co Ltd | Enspryng, エンスプリング | Japan | Neuromyelitis Optica | Chugai Pharmaceutical Co Ltd | 2020-06-29 | Myasthenia Gravis; Brain Ischemia; Graves Ophthalmopathy; Neuromyelitis Optica; Pulmonary Arterial Hypertension; Encephalitis; Subarachnoid Hemorrhage; Anti-N-Methyl-D-Aspartate Receptor Encephalitis | Details |
| Sarilumab | SAR-153191; REGN-88 | Approved | Sanofi | Kevzara | United States | Arthritis, Rheumatoid | Sanofi-Synthelabo | 2017-05-22 | Non-radiographic axial spondyloarthritis; Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19); Sarcoidosis; Mastocytosis, Systemic; Arthritis, Juvenile; Polymyalgia Rheumatica; Severe Acute Respiratory Syndrome; Uveitis | Details |
| Tocilizumab biosimilar (Livzon Group) | LZM-008 | Approved | Livzon Pharmaceutical Group Inc | 安维泰 | Mainland China | Arthritis, Rheumatoid | Livzon Mabpharm Inc | 2023-01-18 | Arthritis, Rheumatoid | Details |
| Tocilizumab biosimilar (Fresenius kabi) | MSB-11456 | Approved | Merck Serono | Tyenne® | EU | Cytokine Release Syndrome; Arthritis, Rheumatoid; Giant Cell Arteritis; Coronavirus Disease 2019 (COVID-19) | Fresenius Kabi Deutschland Gmbh | 2023-09-15 | Cytokine Release Syndrome; Giant Cell Arteritis; Polyarticular Juvenile Idiopathic Arthritis; Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19) | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Recombinant humanized anti-IL-6R antibody (Hisun Pharma) | HS-628 | Phase 3 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Arthritis, Rheumatoid; Arthritis | Details |
| Tocilizumab biosimilar (AryoGen Pharmed) | Phase 3 Clinical | Aryogen Biopharma | Arthritis, Rheumatoid | Details | |
| CMAB806 | CMAB-806 | Phase 3 Clinical | Taizhou Maibo Taike Biotechnology Co Ltd | Arthritis, Rheumatoid; Arthritis | Details |
| Levilimab | BCD-089 | Phase 3 Clinical | Biocad | Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19) | Details |
| Olamkicept | FE-999301; CR-5/18; TJ-301; FE-301 | Phase 2 Clinical | Conaris Research Institute | Colitis, Ulcerative | Details |
| Atexakin alfa | SON-080; r-IL-6 | Phase 2 Clinical | Weizmann Institute Of Science | Peripheral Nervous System Diseases; Diabetic Neuropathies | Details |
| VDJ-001 | Phase 2 Clinical | Beijing Vdjbio Co Ltd | Arthritis, Rheumatoid; Multicentric Castleman's Disease (MCD) | Details | |
| Tocilizumab Biosimilar (qyuns) | QX-003-S | Phase 1 Clinical | Qyuns Therapeutics Co Ltd | Arthritis, Rheumatoid | Details |
| Tocilizumab Biosimilar (Destiny) | IA-001; IA001 | Phase 1 Clinical | Shanghai Destiny Biotech Co Ltd | Polyarticular Juvenile Idiopathic Arthritis; Arthritis, Rheumatoid | Details |
| Recombinant humanized anti-IL-6R antibody (Hisun Pharma) | HS-628 | Phase 3 Clinical | Zhejiang Hisun Pharmaceutical Co Ltd | Arthritis, Rheumatoid; Arthritis | Details |
| Tocilizumab biosimilar (AryoGen Pharmed) | Phase 3 Clinical | Aryogen Biopharma | Arthritis, Rheumatoid | Details | |
| CMAB806 | CMAB-806 | Phase 3 Clinical | Taizhou Maibo Taike Biotechnology Co Ltd | Arthritis, Rheumatoid; Arthritis | Details |
| Levilimab | BCD-089 | Phase 3 Clinical | Biocad | Arthritis, Rheumatoid; Coronavirus Disease 2019 (COVID-19) | Details |
| Olamkicept | FE-999301; CR-5/18; TJ-301; FE-301 | Phase 2 Clinical | Conaris Research Institute | Colitis, Ulcerative | Details |
| Atexakin alfa | SON-080; r-IL-6 | Phase 2 Clinical | Weizmann Institute Of Science | Peripheral Nervous System Diseases; Diabetic Neuropathies | Details |
| VDJ-001 | Phase 2 Clinical | Beijing Vdjbio Co Ltd | Arthritis, Rheumatoid; Multicentric Castleman's Disease (MCD) | Details | |
| Tocilizumab Biosimilar (qyuns) | QX-003-S | Phase 1 Clinical | Qyuns Therapeutics Co Ltd | Arthritis, Rheumatoid | Details |
| Tocilizumab Biosimilar (Destiny) | IA-001; IA001 | Phase 1 Clinical | Shanghai Destiny Biotech Co Ltd | Polyarticular Juvenile Idiopathic Arthritis; Arthritis, Rheumatoid | Details |
This web search service is supported by Google Inc.